Background
The pathophysiology of Sickle Cell Disease (SCD) is characterized by chronic intravascular hemolysis and ischemia-reperfusion, resulting in oxidative stress and chronic inflammation. Although inflammation seems to play an important role in the development of tissue damage in SCD, little is known about the various cellular and cytokine pathways involved in the pathophysiology of SCD. This study aims to further elucidate the inflammatory pathways in SCD using targeted plasma protein profiling both in steady state and during vaso-occlusive crisis (VOC).
Methods
In this prospective study, SCD patients with the genotypes HbSS and HbSβ 0-thalassemia and healthy ethnicity matched controls were included. Plasma levels of 92 proteins involved in various inflammatory pathways were measured in EDTA plasma using Olink Proximity Extension Assay (PEA) technology. In brief, pairs of oligonucleotide-labeled antibody probes were added to 20 μL of plasma, resulting in the binding of these antibody probes to their targeted protein. Subsequently DNA polymerase was added, leading to a proximity-dependent DNA polymerization event, generating a unique PCR target sequence. The final assay read-out is presented in Normalized Protein eXpression (NPX) values, which is an arbitrary unit on a log2-scale with an NPX difference (estimate) of 1 meaning a doubling of protein concentration. RStudio version 4.2.1 was used for data analysis and visualization. Differences between subgroups were tested using an unpaired or paired t-test as appropriate. Results were corrected for multiple testing using the Benjamini-Hochberg method. Results with p < 0.05 were considered significant. Furthermore Over-representation Analysis (ORA) was performed to investigate the association of the differentially expressed proteins with specific biological processes.
Results
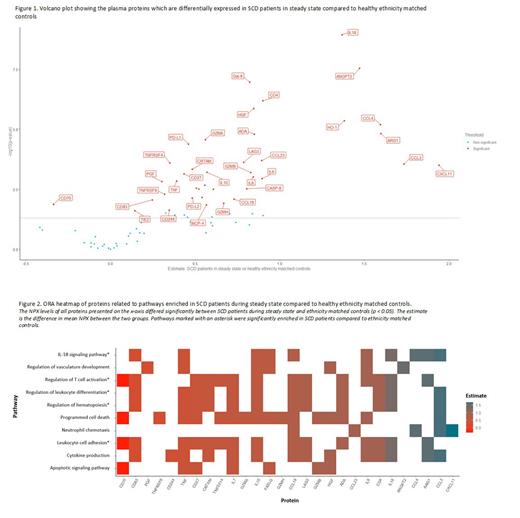
Plasma samples were collected from 43 SCD patients in steady state (median age: 25 years (IQR: 20 - 32)) and 13 healthy ethnicity matched controls (median age: 29 (IQR: 28-39)). From 15 SCD patients plasma samples were collected during both steady state and VOC (median age: 27 (IQR: 19 - 34)). Comparison of protein levels between healthy controls and SCD patients in steady state identified 38 proteins (IL-18, ANGPT2, Gal-9, CD4, HGF, HO-1, CCL4, ARG1, ADA, GZMA, PD-L1, CCL23, LAG3, TNFRSF4, CCL3, CXCL11, CRTAM, IL-10, GZMB, CD27, FGF2, IL-8, IL-6, TNF, PGF, NCR1, TNFSF14, CASP-8, IL-7, FASLG, TNFRSF9, LAP TGF-beta-1, PD-L2, CCL19, CD83, GZMH, MCP-4, CD244, TIE2) that were significantly higher in SCD patients as shown in Figure 1. Furthermore soluble CD70 was significantly lower in SCD patients in steady state compared to healthy controls (estimate: -0.34, p < 0.05). Over-representation Analysis revealed a significant upregulation of proteins involved in the regulation of hematopoiesis, the IL-18 signaling pathway, T-cell activation, leukocyte differentiation and leukocyte adhesion (Figure 2). Pairwise comparison of protein levels between steady state and VOC in SCD patients revealed 6 proteins (TWEAK, VEGFR-2, CD244, PDCD1, GZMA, TNFRSF4) which were significantly lower and 12 proteins (IL-6, TNFSF14, ADA, IL-15, HGF, IL-10, IL-8, Gal-9, CSF-1, CCL20, MMP7, VEGFA) which were significantly higher during VOC as compared to steady state. During VOC, pathways involved in the regulation of cell death (IL-6, TNFSF14, ADA, HGF, IL-10, PDCD1, VEGFA) and tissue development (IL-6, ADA, HGF, IL-10, VEGFA) were significantly enriched.
Summary/conclusion
The results of these proteomic analyses provide new insights into the pathways that regulate inflammation in SCD, both during steady state and VOC. These findings might contribute to a better understanding of SCD pathophysiology and identifying potential new targets for therapeutic interventions in SCD patients. Our results warrant further in-depth investigation of the different activated pathways.
Disclosures
Fijnvandraat:Sanofi: Consultancy; F. Hoffmann-La Roche Ltd: Consultancy; Novo Nordisk: Consultancy, Research Funding; CSL Behring: Research Funding; Sobi: Consultancy, Research Funding. Biemond:CSL Behring: Other: Advisory board; BMS: Research Funding; Global Blood Therapeutics/Pfizer: Other: Advisory board, Research Funding; Novartis: Other: Advisory board, Research Funding; Novo Nordisk: Other: Advisory board; Celgene: Other: Advisory board; Sanquin: Research Funding. Nur:Novartis: Consultancy, Research Funding, Speakers Bureau; VERTEX: Consultancy, Speakers Bureau; Amgen: Consultancy, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal